Minimal Gd Retention
In brain and body tissues5-10*
Safety Profile
Established safety profile in over 40 million patients11
Proven Efficacy
Performances of Gadavist and ProHance for visualization parameters† are similar in MRI of the CNS12,13
*Based on animal studies and small scale clinical investigations. The clinical significance of Gd retention is unknown. Generalizability of animal model results to humans has not been established.
†Visualization parameters include border delineation, information upon internal morphology, and contrast enhancement.
Download your free copy
In this paper, the Barrow Neurological Institute shares:
• Their rationale for switching to a macrocyclic GBCA
• How differences between macrocyclics factored into their choice of agent
• Outcomes and experience with ProHance to date
Gadolinium retention is seen following the administration of any of the GBCAs, but there are differences among linear and macrocyclic agents 5,6,14-16
Levels of retained Gd following macrocyclic GBCAs are very low, showing some degree of variability.
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at four weeks has been established. Gadolinium retention equalizes over time.
Cerebellum
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at four weeks has been established. Gadolinium retention equalizes over time.
Adapted from Bussi et al. Insights into Imaging. 2020. Gd = gadolinium; LOQ = limit of quantitation. Gadovist is the brand name in Europe for Gadavist® (Gadobutrol).
Cerebrum
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at four weeks has been established. Gadolinium retention equalizes over time.
Adapted from Bussi et al. Insights into Imaging. 2020. Gd = gadolinium; LOQ = limit of quantitation. Gadovist is the brand name in Europe for Gadavist® (Gadobutrol).
Liver
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at four weeks has been established. Gadolinium retention equalizes over time.
Adapted from Bussi et al. Insights into Imaging. 2020. Gd = gadolinium; LOQ = limit of quantitation. Gadovist is the brand name in Europe for Gadavist® (Gadobutrol).
Kidneys
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at four weeks has been established. Gadolinium retention equalizes over time.
Adapted from Bussi et al. Insights into Imaging. 2020. Gd = gadolinium; LOQ = limit of quantitation. Gadovist is the brand name in Europe for Gadavist® (Gadobutrol).
Skin
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at four weeks has been established. Gadolinium retention equalizes over time.
Adapted from Bussi et al. Insights into Imaging. 2020. Gd = gadolinium; LOQ = limit of quantitation. Gadovist is the brand name in Europe for Gadavist® (Gadobutrol).
Femur
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at four weeks has been established. Gadolinium retention equalizes over time.
Adapted from Bussi et al. Insights into Imaging. 2020. Gd = gadolinium; LOQ = limit of quantitation. Gadovist is the brand name in Europe for Gadavist® (Gadobutrol).
Data from the Bussi studies in rats using ICP-MS consistently indicate…
ProHance demonstrated low Gd retention in body organs in rat studies.5,6
Cerebellum
Cerebrum
Liver
Kidneys
Skin
Femur
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at four weeks has been established. Gadolinium retention equalizes over time.
Data from the Bussi study, using ICP-MS, indicate…
ProHance demonstrated low Gd retention in body organs in rat study.3
Human data from a small-scale tissue sampling study using ICP-MS indicate that…
ProHance administration results in low residual gadolinium in the brain.7
Adapted from Murata N, et al. Invest Radiol. 2016
Gadovist is the brand name in Europe for Gadavist® (gadobutrol).
Data from extensive animal studies in rats using ICP-MS indicate…
Fast elimination of ProHance in the first 5 weeks after last injection.8,17
Generalizability of animal model results to humans has not been established. Neither the clinical significance of Gd retention in tissues nor the significance of Gd retention differences at five weeks has been established. Gadolinium retention equalizes over time.
Adapted from Jost G, et al. Radiology. 2018
6,163 patients evaluated, 0 serious AEs18
Prospective multicenter data from 6,163 patients who received ProHance demonstrate a 0.16% AE rate, with no serious AEs reported.
28,078 patients and an AE rate of 0.66%19
In a prospective observational study consisting of 28,078 patients exposed to ProHance, there was an overall adverse reaction rate of 0.66%.
Safe for use in children, including term neonates20
Indicated for use in MRI in children, including term neonates, to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine, and associated tissue20
ProHance: The only GBCA with the option for a cumulative dose up to 3 times standard 0.1 mmol/kg dose in adult patients.20
ProHance is not approved for triple dose in children.
Detection and characterization of CNS lesions with ProHance (Gadoteridol) was non-significantly different when compared to Gadavist (gadobutrol)…13
Minimal differences in relaxivity between ProHance and Gadavist do not demonstrate clinical differences in routine neuroradiological applications of CNS MRI.12,13
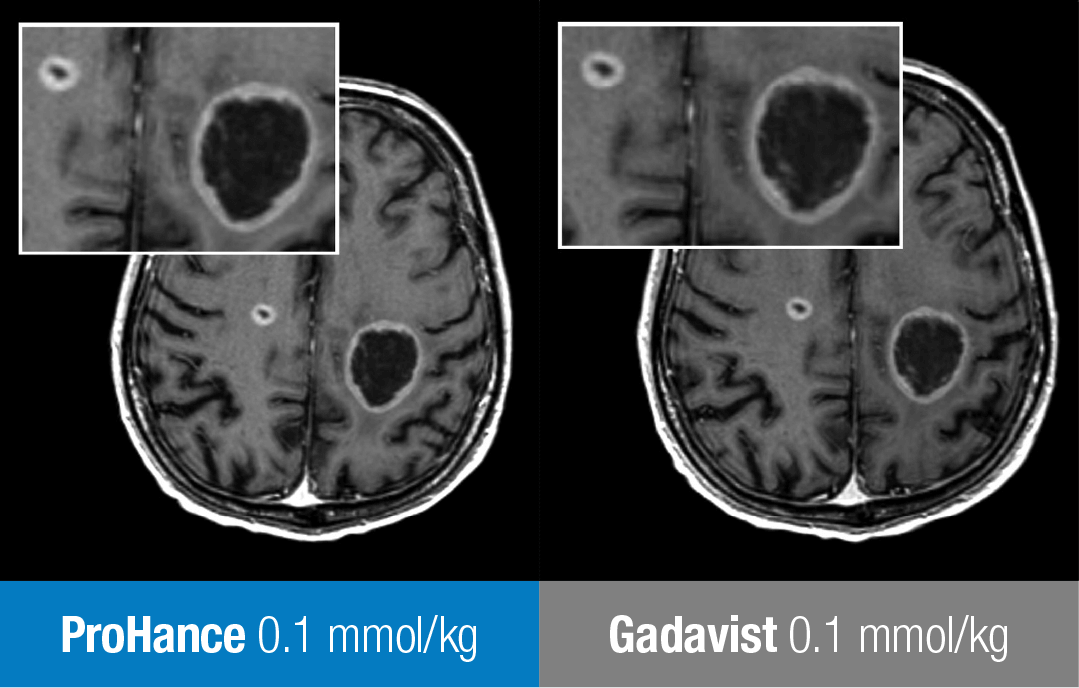
These are representative images from reference studies; individual results may vary. 61-year-old man with brain metastases from primary lung cancer. Two lesions clearly seen in both exams show no differences in contrast enhancement or in the morphology of lesions.13
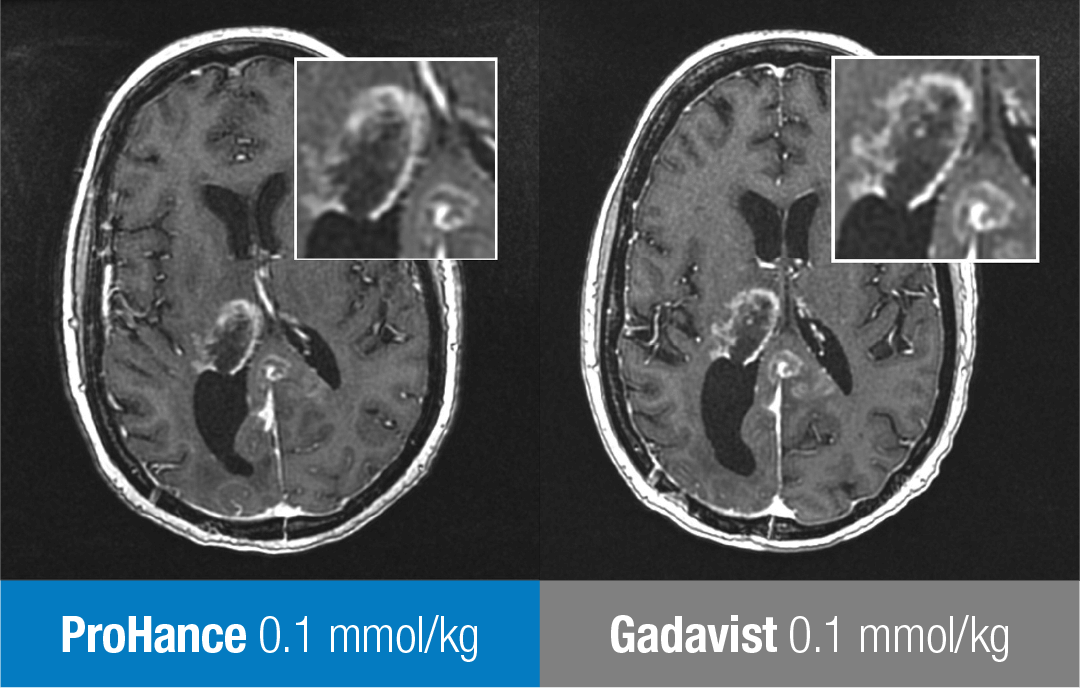
These are representative images from reference studies; individual results may vary. 51-year-old woman with glioblastoma multiforme. Rim-enhancing mass in right thalamus with extension into the posterior interhemispheric region is clearly seen in both examinations. No differences in contrast enhancement or in the morphology of lesions are apparent.13
Adapted from Maravilla KR, et al. AJNR Am J Neuroradiol. 2015
The TRUTH study showed no significant difference by any reader for any of the 5 endpoints:13
• Global diagnostic preference
• Lesion border delineation
• Definition of disease extent
• Visualization of lesion internal morphology
• Lesion contrast enhancement
Study Results: 209 patients successfully completed both examinations. No reader noted a significant qualitative or quantitative difference in lesion enhancement, extent, delineation, or internal morphology (P values .69–1.00). 139 patients had at least 1 histologically confirmed brain lesion. 2 readers found no difference in the detection of patients with lesions (133/139 versus 135/139, P=.317; 137/139 versus 136/139, P=.564), while 1 reader found minimal differences in favor of gadoteridol (136/139 versus 132/139, P=0.46). Similar findings were noted for the number of lesions detected and characterization of tumors (malignant/benign). 3-reader agreement for characterization was similar for gadobutrol (66.4% [κ=0.43]) versus gadoteridol (70.3% [κ=0.45]).
Explore an advanced, high-relaxivity MR agent
Explore reimbursement, savings options, and individualized resources to meet your needs
Learn more about integrating MR suites with SmartInject solutions
Gd = Gadolinium.
GBCA = Gadolinium-based contrast agent.
ICP-MS = Inductively coupled plasma mass spectrometry.
MultiHance® (gadobenate dimeglumine) injection, 529 mg/mL and ProHance® (Gadoteridol) Injection, 279.3 mg/mL
Indications and Usage for MultiHance® (gadobenate dimeglumine) injection, 529 mg/mL:
- Magnetic resonance imaging (MRI) of the central nervous system (CNS) in adults and pediatric patients (including term neonates) to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine, and associated tissues
- Magnetic resonance angiography (MRA) to evaluate adults with known or suspected renal or aorto-ilio-femoral occlusive vascular disease
Indications and Usage for ProHance® (Gadoteridol) Injection, 279.3 mg/mL:
CENTRAL NERVOUS SYSTEM
ProHance is indicated for use in MRI in adults and pediatric patients including term neonates to visualize lesions with disrupted blood brain barrier and/or abnormal vascularity in the brain (intracranial lesions), spine, and associated tissues.
EXTRACRANIAL/EXTRASPINAL TISSUES
ProHance is indicated for use in MRI in adults to visualize lesions in the head and neck.
IMPORTANT SAFETY INFORMATION for MultiHance and ProHance:
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. MultiHance and ProHance are not approved for intrathecal use.
NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs.
- The risk for NSF appears highest among patients with:
- chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
-
For patients at highest risk for NSF, do not exceed the recommended MultiHance and ProHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration.
MultiHance (gadobenate dimeglumine) injection, 529 mg/mL
CONTRAINDICATIONS
MultiHance is contraindicated in patients with known allergic or hypersensitivity reactions to gadolinium-based contrast agents.
WARNINGS AND PRECAUTIONS
Risk Associated with Intrathecal Use: Intrathecal administration of GBCAs can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of MultiHance have not been established with intrathecal use and MultiHance is not approved for intrathecal use.
Nephrogenic Systemic Fibrosis: NSF has occurred in patients with impaired elimination of GBCAs. Higher than recommended dosing or repeated dosing appears to increase risk.
Hypersensitivity Reactions: Anaphylactic and anaphylactoid reactions have been reported, involving cardiovascular, respiratory, and/or cutaneous manifestations. Some patients experienced circulatory collapse and died. In most cases, initial symptoms occurred within minutes of MultiHance administration and resolved with prompt emergency treatment. Consider the risk for hypersensitivity reactions, especially in patients with a history of hypersensitivity reactions or a history of asthma or other allergic disorders.
Gadolinium Retention: Gadolinium is retained for months or years in several organs. The highest concentrations have been identified in the bone, followed by brain, skin, kidney, liver, and spleen. At equivalent doses, retention varies among the linear agents. Retention is lowest and similar among the macrocyclic GBCAs. Consequences of gadolinium retention in the brain have not been established, but they have been established in the skin and other organs in patients with impaired renal function. Minimize repetitive GBCA imaging studies, particularly closely spaced studies when possible.
Acute Renal Failure: In patients with renal insufficiency, acute renal failure requiring dialysis or worsening renal function have occurred with the use of GBCAs. The risk of renal failure may increase with increasing dose of the contrast agent. Screen all patients for renal dysfunction by obtaining a history and/or laboratory tests.
Extravasation and Injection Site Reactions: Extravasation of MultiHance may lead to injection site reactions, characterized by local pain or burning sensation, swelling, blistering, and necrosis. Exercise caution to avoid local extravasation during intravenous administration of MultiHance.
Cardiac Arrhythmias: Cardiac arrhythmias have been observed in patients receiving MultiHance in clinical trials. Assess patients for underlying conditions or medications that predispose to arrhythmias. The effects on QTc by MultiHance dose, other drugs, and medical conditions were not systematically studied.
Interference with Visualization of Certain Lesions: Certain lesions seen on non-contrast images may not be seen on contrast images. Exercise caution when interpreting contrast MR images in the absence of companion non-contrast MR images.
ADVERSE REACTIONS
The most commonly reported adverse reactions are nausea (1.3%) and headache (1.2%).
USE IN SPECIFIC POPULATIONS
Pregnancy: GBCAs cross the human placenta and result in fetal exposure and gadolinium retention. Use only if imaging is essential during pregnancy and cannot be delayed.
Lactation: There is no information on the effects of the drug on the breastfed infant or the effects of the drug on milk production. However, limited literature reports that breastfeeding after MultiHance administration to the mother would result in the infant receiving an oral dose of 0.001%-0.04% of the maternal dose.
Pediatric Use: MultiHance is approved for intravenous use for MRI of the CNS to visualize lesions with abnormal blood brain barrier or abnormal vascularity of the brain, spine, and associated tissues in pediatric patients from birth, including term neonates, to less than 17 years of age. Adverse reactions in pediatric patients were similar to those reported in adults. No dose adjustment according to age is necessary in pediatric patients two years of age and older. For pediatric patients, less than 2 years of age, the recommended dosage range is 0.1 to 0.2 mL/kg. The safety of MultiHance has not been established in preterm neonates.
ProHance (Gadoteridol) Injection, 279.3 mg/mL
CONTRAINDICATIONS
Contraindicated in patients with known allergic or hypersensitivity reactions to ProHance.
WARNINGS AND PRECAUTIONS
Risk Associated with Intrathecal Use: Intrathecal administration of GBCAs can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of ProHance have not been established with intrathecal use and ProHance is not approved for intrathecal use.
Nephrogenic Systemic Fibrosis: NSF has occurred in patients with impaired elimination of GBCAs. Higher than recommended dosing or repeated dosing appears to increase risk.
Hypersensitivity Reactions: Anaphylactic and anaphylactoid reactions have been reported, involving cardiovascular, respiratory, and/or cutaneous manifestations. Some patients experienced circulatory collapse and died. In most cases, initial symptoms occurred within minutes of administration and resolved with prompt emergency treatment. Prior to ProHance administration, ensure the availability of trained personnel and medications to treat hypersensitivity reactions. Consider these risks, especially in patients with a history of hypersensitivity reactions or a history of asthma or other allergic disorders.
Gadolinium Retention: Gadolinium is retained for months or years in several organs. The highest concentrations have been identified in the bone, followed by brain, skin, kidney, liver, and spleen. Linear GBCAs cause more retention than macrocyclic GBCAs. Consequences of gadolinium retention in the brain have not been established, but they have been established in the skin and other organs in patients with impaired renal function.
Acute Kidney Injury: In patients with chronically reduced renal function, acute kidney injury requiring dialysis has occurred with the use of GBCAs. The risk of acute kidney injury may increase with increasing dose of the contrast agent; administer the lowest dose necessary for adequate imaging.
ADVERSE REACTIONS
The most commonly reported adverse reactions are nausea and taste perversion with an incidence ≥ 0.9%.
USE IN SPECIFIC POPULATIONS
Pregnancy: GBCAs cross the human placenta and result in fetal exposure and gadolinium retention. Use only if imaging is essential during pregnancy and cannot be delayed.
Lactation: There are no data on the presence in human milk, the effects on the breastfed infant, or the effects on milk production. However, published lactation data on other GBCAs indicate that 0.01 to 0.04% of the maternal gadolinium dose is present in breast milk.
Pediatric Use: The safety and effectiveness of ProHance have been established for use with MRI to visualize lesions with abnormal blood brain barrier or abnormal vascularity of the brain, spine, and associated tissues in pediatric patients from birth, including term neonates, to 17 years of age. Adverse reactions in pediatric patients were similar to those reported in adults. No case of NSF associated with ProHance or any other GBCA has been identified in pediatric patients ages 6 years and younger.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1‑800‑FDA‑1088.
Please click here for full Prescribing Information and Patient Medication Guide for additional safety information for MultiHance (gadobenate dimeglumine) injection, 529 mg/mL.
Please click here for full Prescribing Information and Patient Medication Guide for additional safety information for MultiHance Multipack.
Please click here for full Prescribing Information and Patient Medication Guide for additional safety information for ProHance (Gadoteridol) Injection, 279.3 mg/mL.
Please click here for full Prescribing Information and Patient Medication Guide for additional safety information for ProHance Multipack.
MultiHance is manufactured for Bracco Diagnostics Inc. by BIPSO GmbH – 78224 Singen (Germany) and by Patheon Italia S.p.A, Ferentino, Italy.
ProHance is manufactured for Bracco Diagnostics Inc. by BIPSO GmbH – 78224 Singen (Germany).
MultiHance is a registered trademark of Bracco International B.V.
MultiHance Multipack is a trademark of Bracco International B.V.
ProHance is a registered trademark of Bracco Diagnostics Inc.
ProHance Multipack is a trademark of Bracco Diagnostics Inc.
All other trademarks and registered trademarks are the property of their respective owners.
References:
- Idée JM, Port M, Raynal I, et al. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol. 2006 Jun; 20(6): 563–576.
- Laurent S, Elst LV, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol Imaging. 2006 Feb; 1(3): 128–137.
- Idée JM, Port M, Robic C, et al. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009 Dec; 30(6): 1249–1258.
- Gianolio E, Bardini P, Arena F, et al. Gadolinium retention in the rat brain: assessment of the amounts of insoluble gadolinium-containing species and intact gadolinium complexes after repeated administration of gadolinium-based contrast agents. Radiology. 2017 Sep; 285(3): 839–849.
- Bussi S, Coppo A, Celeste R, et al. Macrocyclic MR contrast agents: evaluation of multiple-organ gadolinium retention in healthy rats. Insights Imaging. 2020 Feb;11(11):doi.org/10.1186/s13244-019-0824-5.
- Bussi S, Coppo A, Botteron C, et al. Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J Magn Reson Imaging. 2018 Mar;47(3):746–752. (Macrocyclic MR contrast agents administered to rats over a 5 week period and Gd retention tested 4 weeks after last administration.)
- Murata N, Gonzalez-Cuyar LF, Murata K, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016 Jul;51(7):447–453. (Tissue samples were collected from 9 decedents undergoing autopsy who had contrast-enhanced magnetic resonance imaging (MRI) with only single agent exposure to a non-Group 1 Gd-based contrast agent.)
- Jost G, Frenzel T, Boyken J, et al. Long-term excretion of gadolinium-based contrast agents: linear versus macrocyclic agents in an experimental rat model. Radiology. 2019 Feb;290(2):340–348. doi: 10.1148/radiol.2018180135. Epub 2018 Nov 13. (Linear and macrocyclic MR contrast agents administered to rats over a 2 week period and Gd retention tested 5, 26, and 52 weeks after administration.)
- Frenzel T, Ulbrich HF, Pietsch H. The macrocyclic gadolinium-based contrast agents gadobutrol and gadoteridol show similar elimination kinetics from the brain after repeated intravenous injections in rabbits. Invest Radiol. 2021 Jun;56(6):341–347. doi: 10.1097/RLI.0000000000000749.
- Radbruch A, Richter H, Fingerhut S, et al. Gadolinium deposition in the brain in a large animal model: comparison of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol. 2019 Sep;54(9):531–536.
- Data on file. Bracco Diagnostics Inc. based on IQVIA DDD: January 2021.
- Gadavist® (gadobutrol) injection full Prescribing Information and Patient Medication Guide. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.; April 2022.
- Maravilla KR, Smith MP, Vymazal J, et al. Are there differences between macrocyclic gadolinium contrast agents for brain tumor imaging? Results of a multicenter intraindividual crossover comparison of gadobutrol with gadoteridol (the TRUTH study). AJNR Am J Neuroradiol. 2015 Jan;36(1):14–23.
- McDonald RJ, McDonald JS, Dai D, et al. Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology. 2017 Nov; 285(2):536–545. (Linear and macrocyclic MR contrast agents administered to rats over a 26 day period and Gd retention tested 7 days after final administration.)
- McDonald RJ, Levine D, Weinreb J, et al. Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018 Nov;289(2):517–534. doi: 10.1148/radiol.2018181151. Epub 2018 Sep 11.
- Guo BJ, Yang ZL, Zhang LJ. Gadolinium deposition in brain: current scientific evidence and future perspectives. Front Mol Neurosci. 2018 Sep 20;11:335. doi: 10.3389/fnmol.2018.00335. (Review article.)
- Bussi S, Coppo A, Bonafè R, et al. Gadolinium clearance in the first 5 weeks after repeated intravenous administration of gadoteridol, gadoterate meglumine, and gadobutrol to rats. J Magn Reson Imaging. 2021 Nov;54(5):1636–1644. doi: 10.1002/jmri.27693. Epub 2021 May 11.
- Cho SB, Lee Al, Chang HW, et al. Prospective multicenter study of the safety of gadoteridol in 6163 patients. J Magn Reson Imaging. 2020 Mar;51(3):861–868. doi:10.1002/jmri.26940.
- Morgan DE, Spann JS, Lockhart ME, et al. Assessment of adverse reaction rates during gadoteridol enhanced MR imaging in 28,078 patients. Radiology. 2011 Apr;259(1):109–116. doi: 10.1148/radiol.10100906. Epub 2011 Jan 19.
- ProHance® (Gadoteridol) Injection, 279.3 mg/mL Full Prescribing Information and Patient Medication Guide. Monroe Twp., NJ: Bracco Diagnostics Inc.; January 2024.
IMPORTANT SAFETY INFORMATION for MultiHance® (gadobenate dimeglumine) injection, 529 mg/mL and ProHance® (Gadoteridol) Injection, 279.3 mg/mL
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. MultiHance and ProHance are not approved for intrathecal use.
IMPORTANT SAFETY INFORMATION for MultiHance® (gadobenate dimeglumine) injection, 529 mg/mL and ProHance® (Gadoteridol) Injection, 279.3 mg/mL
|
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs. |